Determination of starane (fluroxypyr) herbicide using flow injection spectrophotometry.
Jasmin Shah, M Rasul Jan, Nadia Bashir
Index: Anal. Sci. 22(1) , 145-6, (2006)
Full Text: HTML
Abstract
The starane herbicide was spectrophotometrically determined by the diazotization method in a flow injection assembly. Since starane is a substituted pyridyl compound the NH2 group at the p-position was exploited for diazotization. Starane was diazotized with nitrite and the diazotized product is coupled with beta-naphthol. The absorbance of the resulting azo dye was measured at 395 nm with a molar absorptivity of 1.5 x 10(4) L mol(-1) cm(-1). The calibration graph was linear over the range of 0.6 to 10 microg/mL, with a relative standard deviation (RSD) of 1.67% and a sampling through put of 60 samples h(-1). The % recovery for the determination of starane was found to be 96%. The method was successfully applied to the determination of the active ingredient of starane herbicide in its formulation as well as in food samples.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
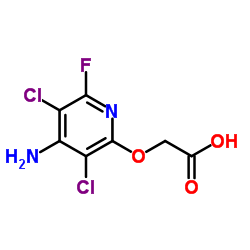 |
Fluroxypyr
CAS:69377-81-7 |
C7H5Cl2FN2O3 |
|
Method development for determination of fluroxypyr in water.
2003-07-01 [J. Environ. Sci. Health B 38(4) , 429-40, (2003)] |
|
A pathway-specific microarray analysis highlights the comple...
2009-01-01 [J. Exp. Bot. 60 , 153-67, (2009)] |
|
[Determination of fluroxypyr residues in garlic and corn by ...
2004-01-01 [Se Pu 22(1) , 92, (2004)] |
|
Study of the selectivity of PRIMSTAR (fluroxypyr + florasula...
2003-01-01 [Commun. Agric. Appl. Biol. Sci. 68(4 Pt A) , 381-90, (2003)] |
|
[Toxicity and hazards of the herbicide starane 200].
2000-01-01 [Gig. Sanit. (5) , 59-60, (2000)] |