Conversion of a hemoglobin alpha chain aspartate(47) ester to N-(2,3-dihydroxypropyl)asparagine as a method for identification of the principal binding site for benzo[a]pyrene anti-diol epoxide.
B W Day, P L Skipper, R H Rich, S Naylor, S R Tannenbaum
Index: Chem. Res. Toxicol. 4(3) , 359-63, (1991)
Full Text: HTML
Abstract
Human hemoglobin was alkylated with (+/-)-7 beta,8 alpha-dihydroxy-9 alpha,10 alpha-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene (BPDE) and then treated with aqueous (+/-)-3-amino-1,2-propanediol to convert alkylated carboxyl side chains to N-(2,3-dihydroxypropyl) amides. Tryptic peptides produced from the modified protein were subjected to affinity chromatography on phenylboronic acid. The bound fraction was further purified by HPLC on C-4 reverse-phase medium to yield one modified peptide, which was identified as the Thr(41)-Lys(56) peptide of the alpha chain by amino acid analysis, Edman sequencing analysis, and FAB-MS. Limited direct evidence from this study and further indirect evidence from previous work identify Asp(47) alpha as the amino acid reacting with BPDE. The only other likely sites would be the C-terminal carboxyl groups of either the alpha or beta chain. Possible reasons for the site selectivity of the alkylation of human hemoglobin by BPDE are discussed.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
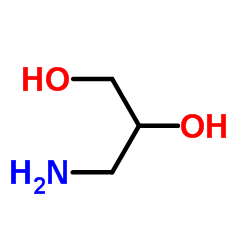 |
3-Amino-1,2-propanediol
CAS:616-30-8 |
C3H9NO2 |
|
Synthesis and properties of DNA-PNA chimeric oligomers.
1996-09-01 [Nucleic Acids Res. 24(17) , 3357-63, (1996)] |
|
Competitive inhibition of lipolytic enzymes. II. Preparation...
1990-03-12 [Biochim. Biophys. Acta 1043(1) , 67-74, (1990)] |
|
Three new derivatives of 3-amino-1,2-propanediol; their spec...
2001-01-01 [Acta Pol. Pharm. 58(4) , 249-56, (2001)] |
|
A specific receptor site for glycerol, a new sweet tastant f...
2004-10-01 [Chem. Senses 29(8) , 703-11, (2004)] |
|
A new nanoprobe based on FRET between functional quantum dot...
2012-07-01 [Biosens. Bioelectron. 36(1) , 168-73, (2012)] |