Separation study of cadmium as CdI4(2-) through a bulk liquid membrane containing ketoconazole and oleic acid.
Khalil Farhadi, Mojtaba Shamsipur
Index: Anal. Sci. 21(5) , 501-5, (2005)
Full Text: HTML
Abstract
A chloroform membrane system containing a given mixture of ketoconazole and oleic acid was applied for the uphill transport of Cd2+ ions as CdI42-. In an HCl medium the ligand could form a stable ion-pair with CdI4(2-), which was readily extractable in the membrane phase. A weak basic solution (pH 8) was used as a suitable stripping medium for the quantitative transport of cadmium across the liquid membrane after 120 min. The selectivity and efficiency of Cd2+ transport from an aqueous solution containing other cations, such as Co2+, Cr3+, Ni2+, Fe2+, Mn2+, Pd2+ and Zn2+ ions, were investigated. It was found that none of these cations interfered with Cd2+ transport.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
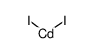 |
Cadmium iodide
CAS:7790-80-9 |
CdI2 |
|
Unprecedented marriage of a cationic pentanuclear cluster an...
2010-02-01 [Inorg. Chem. 49(3) , 769-71, (2010)] |
|
[The formation of an adaptive genetic system in an inbred st...
1991-01-01 [Tsitol. Genet. 25(4) , 67-72, (1991)] |
|
Separation/preconcentration and determination of cadmium ion...
2009-09-15 [Talanta 79(4) , 1061-5, (2009)] |
|
Host dependence of spectroscopic properties of Dy 3+ - doped...
2009-08-17 [Opt. Express 17(17) , 15350-8, (2009)] |
|
[Precipitation of cytochromes c with complex cadmium iodide]...
1996-01-01 [Bioorg. Khim. 22(10-11) , 870-4, (1996)] |