| Structure | Name/CAS No. | Articles |
|---|---|---|
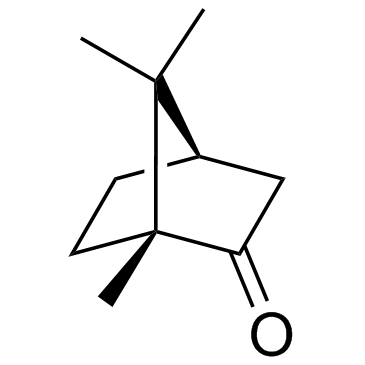 |
(+)-Camphor
CAS:464-49-3 |
|
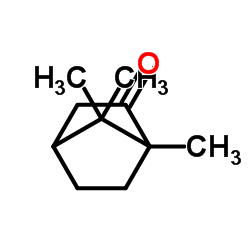 |
(±)-Camphor
CAS:464-48-2 |
|
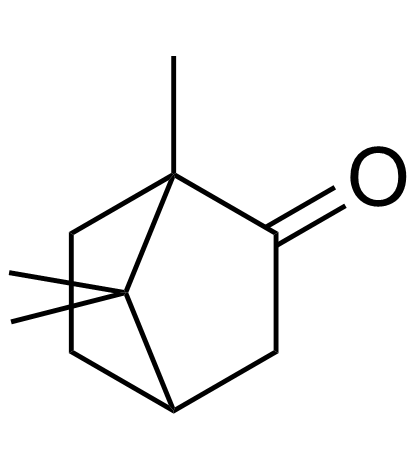 |
(+/-)-Camphor
CAS:76-22-2 |
|
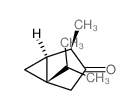 |
α-Thujone
CAS:546-80-5 |