| Structure | Name/CAS No. | Articles |
|---|---|---|
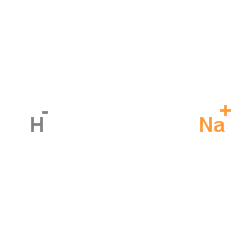 |
Sodium hydride
CAS:7646-69-7 |
|
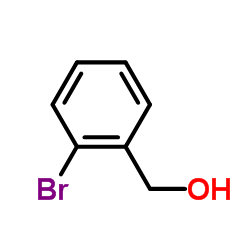 |
2-Bromobenzyl alcohol
CAS:18982-54-2 |
|
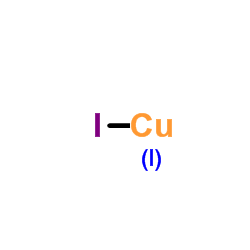 |
Copper(I) iodide
CAS:7681-65-4 |