Adsorption of an azo dye in an aqueous solution using hydroxyl-terminated polybutadiene (HTPB).
Mohammad Ebrahim Olya, Azam Pirkarami, Mohammad Mirzaie
Index: Chemosphere 91(7) , 935-40, (2013)
Full Text: HTML
Abstract
This paper reports an investigation into the effect of a number of operating factors on the removal of Acid Blue 92 (AB92) from an aqueous solution using hydroxyl-terminated polybutadiene (HTPB) as an adsorbent. The optimum values of adsorbent dose and pH were found to be 35mgL(-1) and 6, respectively. Temperature showed a significant effect, with maximum dye removal being observed at 45°C. Stirring the solution during the treatment process resulted in significant removal improvement. The Langmuir adsorption model was used to quantify the amount of AB92 adsorbed on the surface of HTPB. FT-IR spectrometry results for HTPB, AB92, and HTPB-AB92 verified the efficiency of the treatment. Further, the adsorbent was characterized using SEM and H NMR techniques.Copyright © 2013 Elsevier Ltd. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
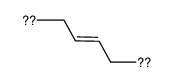 |
polybutadiene diacrylate
CAS:9003-17-2 |
C4H6 |
|
Modifying the response of a polymer-based quartz crystal mic...
2011-09-15 [Talanta 85(3) , 1648-57, (2011)] |
|
Direct synthesis of inverse hexagonally ordered diblock copo...
2012-08-01 [J. Am. Chem. Soc. 134(30) , 12685-92, (2012)] |
|
Core-crosslinked compartmentalized cylinders.
2011-01-01 [Nanoscale 3(1) , 288-97, (2011)] |
|
Migration kinetics and mechanisms of plasticizers, stabilize...
2012-08-30 [J. Hazard. Mater. 229-230 , 251-7, (2012)] |
|
Study of the interaction polybutadiene/fillers using inverse...
2012-08-31 [J. Chromatogr. A. 1253 , 164-70, (2012)] |