| Structure | Name/CAS No. | Articles |
|---|---|---|
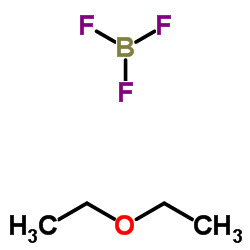 |
Boron trifluoride etherate
CAS:109-63-7 |
|
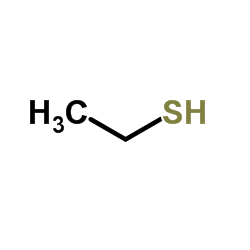 |
Ethanethiol
CAS:75-08-1 |
|
 |
Sodium ethanethiolate
CAS:811-51-8 |
|
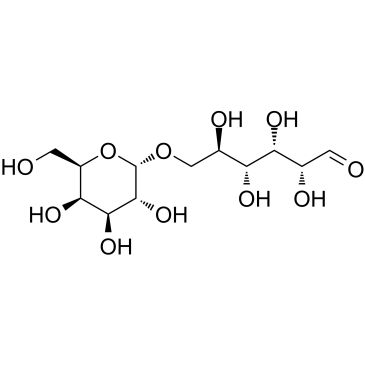 |
Melibiose
CAS:585-99-9 |