Modeling the solvation of peptides. The case of (s)-N-acetylproline amide in liquid water.
Chiara Cappelli, Benedetta Mennucci
Index: J. Phys. Chem. B 112(11) , 3441-50, (2008)
Full Text: HTML
Abstract
The structure and properties of (s)-N-acetylproline amide (NAP) in aqueous solution are studied by exploiting a continuum solvation model. The conformational preference of NAP as a function of the environment is discussed as well as data for a number of chiral and non-chiral spectroscopic and response properties (IR/VCD, Raman/VROA, UV/CD, ORD, NMR), whose calculation with the accounting of solvent effects is now possible due to recent developments introduced in the PCM approach. When available, calculated results are compared with experimental data, so as to evaluate the quality of the continuum approach to the solvation of this system.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
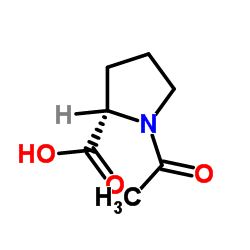 |
N-acetyl-L-proline
CAS:68-95-1 |
C7H11NO3 |
|
Quantum mechanical and NMR studies of ring puckering and cis...
2009-10-08 [J. Phys. Chem. A 113(40) , 10858-65, (2009)] |
|
Angiotensin-converting enzyme inhibitors. Mercaptan, carboxy...
1988-06-01 [J. Med. Chem. 31 , 1148-1160, (1988)] |
|
Synergistic binding of ligands to angiotensin-converting enz...
1988-03-25 [J. Biol. Chem. 263 , 4056-4068, (1988)] |
|
Conformational analysis of L-prolines in water.
2007-12-20 [J. Phys. Chem. B 111 , 14034-14042, (2007)] |
|
Purification and characterization of a novel aminoacylase fr...
2005-10-01 [Biosci. Biotechnol. Biochem. 69 , 1914-1922, (2005)] |