Diastereoselective one-pot tandem synthesis of 3-substituted isoindolinones: a mechanistic investigation.
Marcus Angelin, Martin Rahm, Andreas Fischer, Tore Brinck, Olof Ramström
Index: J. Org. Chem. 75(17) , 5882-7, (2010)
Full Text: HTML
Abstract
The mechanism of a base-catalyzed one-pot reaction of 2-cyanobenzaldehyde and primary nitroalkanes, to produce 3-substituted isoindolinones, has been investigated. A route starting with a nitroaldol (Henry) reaction, followed by a subsequent cyclization and rearrangement, was supported by intermediate analogue synthesis and DFT calculations. Direct diastereoselective crystallization from the reaction mixture was also achieved and studied for a number of substrates. Furthermore, the 3-substituted isoindolinones are an interesting group of compounds, both present important natural products, as well as being precursors to other valuable building blocks.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
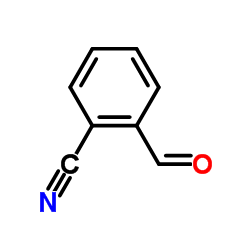 |
2-Cyanobenzaldehyde
CAS:7468-67-9 |
C8H5NO |
|
Bifunctional phase-transfer catalysis in the asymmetric synt...
2015-01-01 [Beilstein J. Org. Chem. 11 , 2591-9, (2015)] |
|
The aldol addition of readily enolizable 1, 3-dicarbonyl com...
[Synthesis 2011(18) , 3027-31, (2011)] |
|
Application of baylis-hillman methodology in a new synthesis...
[J. Heterocycl. Chem. 40(5) , 939-42, (2003)] |
|
Biotransformation of aromatic aldehydes and related compound...
[Phytochemistry 30(9) , 2969-72, (1991)] |
|
Novel synthesis of 3-(N-substituted amino)-1-isoindolenones ...
[Chem. Lett. 9 , 1599-1602, (1984)] |