Facile small scale synthesis of nucleoside 5'-phosphate mixtures.
Robert S Jansen, Hilde Rosing, Jan H M Schellens, Jos H Beijnen
Index: Nucleosides Nucleotides Nucleic Acids 29(1) , 14-26, (2010)
Full Text: HTML
Abstract
We present a facile method to phosphorylate small amounts of nucleosides (0.05 mumol) into mixtures of their 5'-mono-, di-, and triphosphates in a one-pot reaction. The nucleosides were first converted into their dichlorophosphates using a large excess (15-18 equivalents) of phosphorous oxychloride in trimethylphosphate. The large excess resulted in good dichlorophosphate yields (46-76%) for the four nucleosides tested. Upon the addition of tributylammonium-phosphate with additional tributylamine (20 equivalents both), the dichlorophosphate was converted into a mixture containing equal amounts of the mono-, di-, and triphosphate. The presented method was successfully applied to synthesize mixtures of stable isotope labeled nucleotides, which can be used as internal standards in quantitative mass spectrometric assays.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
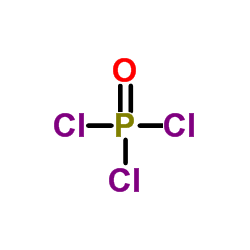 |
phosphoryl trichloride
CAS:10025-87-3 |
Cl3OP |
|
Design of donor-acceptor star-shaped oligomers for efficient...
2014-01-01 [Faraday Discuss. 174 , 313-39, (2014)] |
|
Toughened hydrogels inspired by aquatic caddisworm silk.
2015-09-21 [Soft Matter 11 , 6981-90, (2015)] |
|
Differentiation between acetylcholinesterase and the organop...
2003-11-14 [J. Biol. Chem. 278(46) , 45512-8, (2003)] |
|
POCl3 chlorination of 4-quinazolones.
2011-03-18 [J. Org. Chem. 76(6) , 1653-61, (2011)] |
|
Formation of the Vilsmeier-Haack complex: the performance of...
2011-12-01 [J. Mol. Model. 17(12) , 3209-17, (2011)] |