| Structure | Name/CAS No. | Articles |
|---|---|---|
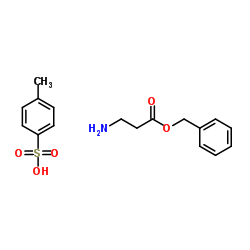 |
beta-Alanine benzyl ester p-toluenesulfonate salt
CAS:27019-47-2 |
| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
beta-Alanine benzyl ester p-toluenesulfonate salt
CAS:27019-47-2 |