The action of the systemic fungicides tridemorph and fenpropimorph on sterol biosynthesis by the soil amoeba Acanthamoeba polyphaga.
D Raederstorff, M Rohmer
Index: Eur. J. Biochem. 164(2) , 421-6, (1987)
Full Text: HTML
Abstract
Tridemorph and fenpropimorph, two systemic fungicides known by their inhibitory effects on sterol biosynthesis in fungi and plants, were administered in vivo to the amoeba Acanthamoeba polyphaga. The compounds did not kill the cells, but modified completely their sterol pattern. Fungicide-exposed cells accumulated cyclopropylsterols indicating a partial blockage of the cyclopropane isomerase as in higher plants and delta 8-sterols indicating an inhibition of the delta 8----delta 7 isomerase as in fungi. Three new sterols, 4 alpha-methylergosta-9(11),24(28)-dienol, ergosta-6,8,22-trienol and poriferasta-6,8,22-trienol were isolated and identified, the former from control cells, the two latter from fungicide-treated cells. These results are in accordance with our previous results on the presence of cycloartenol as sterol precursor and confirm our hypothesis on a phylogenetic relationship of Acanthamoeba polyphaga with photosynthetic phyla.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
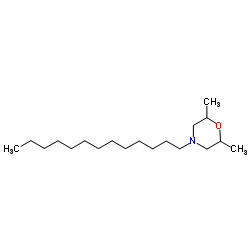 |
Tridemorph
CAS:24602-86-6 |
C19H39NO |
|
Effect of some sterol-biosynthesis-inhibiting fungicides on ...
1989-01-01 [Steroids 53(3-5) , 393-412, (1989)] |
|
[Teratogenic effect of the fungicide calixin].
1981-01-01 [Vopr. Pitan. (6) , 55-61, (1981)] |
|
Human lamin B receptor exhibits sterol C14-reductase activit...
1998-06-15 [Biochim. Biophys. Acta 1392(2-3) , 233-44, (1998)] |
|
Inhibition of 2,3-oxidosqualene: beta-amyrin-cyclase, S-aden...
1983-10-01 [Biochem. Soc. Trans. 11(5) , 537-43, (1983)] |
|
Synthesis and structure revision of calyxin natural products...
2006-04-14 [J. Org. Chem. 71(8) , 3176-83, (2006)] |