Time-dependent enzyme activity dominated by dissociation of J-aggregates bound to protein surface.
Kenji Watanabe, Koji Kano
Index: Bioconjug. Chem. 21(12) , 2332-8, (2010)
Full Text: HTML
Abstract
J-Aggregates of diprotonated 5,10,15,20-tetrakis(4-sulfonatopheny)porphyrin (H₄TPPS²⁻) were stabilized even in a neutral aqueous solution (pH 7.0) containing per-O-methylated β-cyclodextrin by binding to the surface of α-chymotrypsin (ChT). The large J-aggregates covered the active site of ChT and completely inhibited the hydrolysis of the peptides. However, enzyme activity was gradually restored with the dissociation of the J-aggregates attached to the protein surface to monomers. After the completion of dissociation of the aggregates, the enzyme activity was almost completely restored, though the structure of ChT significantly changed. Circular dichroism spectroscopy suggested that the microscopic structure at the active site of ChT was scarcely affected by the J-aggregates, but the binding of J-aggregates to ChT increased the content of the random coils in the enzyme. The present study showed a new type of effector for controlling the function of ChT.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
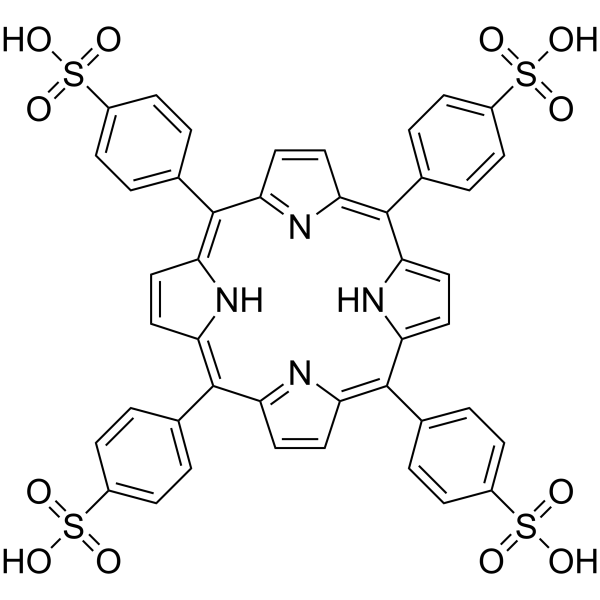 |
Tetraphenylporphyrin Tetrasulfonic Acid Hydrate
CAS:35218-75-8 |
C44H30N4O12S4 |
|
Femtosecond to second studies of a water-soluble porphyrin d...
2012-03-06 [Langmuir 28(9) , 4363-72, (2012)] |
|
Preferential solvation and solvation shell composition of fr...
2011-12-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 83(1) , 213-20, (2011)] |
|
Interaction of monosulfonate tetraphenyl porphyrin (H2TPPS1)...
2011-05-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 78(5) , 1349-55, (2011)] |
|
Evanescent wave cavity ringdown spectroscopy: a platform for...
2012-03-06 [Anal. Chem. 84(5) , 2585-91, (2012)] |
|
Kinetic effects of tartaric acid on the growth of chiral J-a...
2012-05-18 [Chem. Commun. (Camb.) 48(40) , 4872-4, (2012)] |