| Structure | Name/CAS No. | Articles |
|---|---|---|
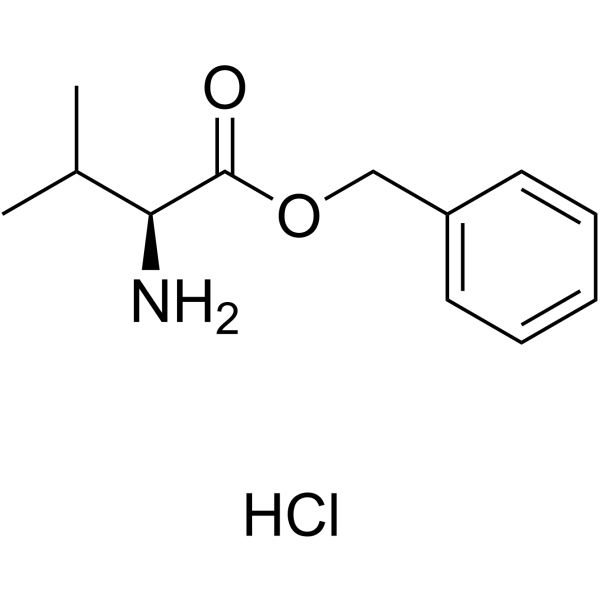 |
Val-OBzl HCl
CAS:2462-34-2 |
|
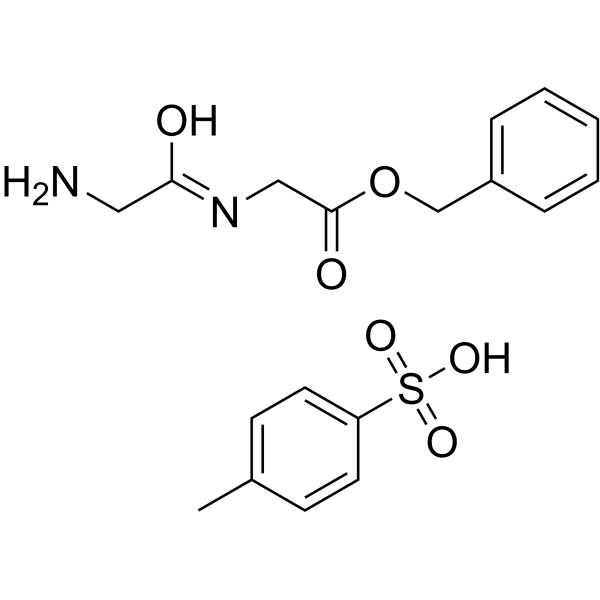 |
H-Gly-Gly-OBzl · p-tosylate
CAS:1738-82-5 |