Effect of add-on pentoxifylline on proteinuria in membranous glomerulonephritis: a 6-month placebo-controlled trial.
Shirinsadat Badri, Simin Dashti-Khavidaki, Farrokhlegha Ahmadi, Mitra Mahdavi-Mazdeh, Mohammad-Reza Abbasi, Hossein Khalili
Index: Clin. Drug Investig. 33(3) , 215-22, (2013)
Full Text: HTML
Abstract
Membranous glomerulonephritis (MGN) may cause proteinuria as the main complication and is a strong risk factor for end-stage renal disease. Current therapeutic regimens provide only partial renoprotection. Data derived from both animal and human studies provide a scientific basis for the use of pentoxifylline as an antiproteinuric agent.This study was designed to evaluate the antiproteinuric effect of add-on pentoxifylline therapy in non-diabetic patients with MGN.This was a double-blind, placebo-controlled trial.Non-diabetic patients with histologically proven MGN and urinary protein excretion (UPE) > 500 mg/24 h, entered a 6-month study period. Enrolled patients were selected from a university and three private clinics.Patients were assigned to one of the two treatment groups: pentoxifylline 400 mg two or three times a day, or matching placebo.Baseline and follow-up assessments included estimated glomerular filtration rate (eGFR) and UPE. Differences in the changes in variables within the placebo and pentoxifylline treatment groups during the study period were assessed using Friedman's test.Treatment with pentoxifylline for 6 months resulted in a significant reduction of mean UPE (p < 0.001) along with a slight, non-significant increase of eGFR, in comparison to the mean UPE and eGFR increase in the placebo group.This study showed that add-on therapy of pentoxifylline in MGN was beneficial, and could be considered as a potential new therapeutic indication for the drug in such kidney diseases.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
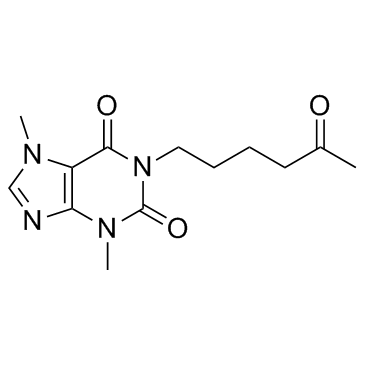 |
Pentoxifylline
CAS:6493-05-6 |
C13H18N4O3 |
|
Modulation of cytochrome P450 2A5 activity by lipopolysaccha...
2015-01-01 [PLoS ONE 10(1) , e0117842, (2015)] |
|
Plasma matrix metalloproteinase activity in horses after int...
2013-03-01 [Am. J. Vet. Res. 74(3) , 473-80, (2013)] |
|
Pentoxifylline for slow to resolve hepatopulmonary syndrome ...
2013-03-01 [Acta Gastroenterol. Belg. 76(1) , 70-1, (2013)] |
|
[Current approach to therapy for male infertility in patient...
2012-01-01 [Ter. Arkh. 84(10) , 56-61, (2012)] |
|
Preclinical evaluation of the antimetastatic efficacy of Pen...
2012-12-01 [Biomed. Pharmacother. 66(8) , 617-26, (2012)] |