Solid-phase extraction for the high-performance liquid chromatographic determination of indomethacin, suxibuzone, phenylbutazone and oxyphenbutazone in plasma, avoiding degradation of compounds.
M C Caturla, E Cusido
Index: J. Chromatogr. A. 581(1) , 101-7, (1992)
Full Text: HTML
Abstract
A solid-phase extraction method was validated for the simultaneous high-performance liquid chromatographic determination of indomethacin, suxibuzone, phenylbutazone, oxyphenbutazone and their degradation products. Indomethacin was added as internal standard to plasma samples, which were then acidified with citrate buffer and passed through a phenyl cartridge. The drugs were eluted with hexane-diethyl ether (1:1, v/v), and the organic extract was taken to dryness. The residue was dissolved in methanol and chromatographed on a C18 column with ultraviolet detection at 240 nm. The elution was isocratic with a mobile phase of 0.02 M ammonium sulphate-acetonitrile (45:55, v/v), pH 3. For indomethacin, suxibuzone, phenylbutazone and oxyphenbutazone the limit of quantitation was 0.05 microgram/ml. The linearity was checked between 0.05 and 100 micrograms/ml (r = 0.999); within this range the recovery was higher than 90% and the accuracy showed relative errors of less than 7.5%. The main advantage of this method is the avoidance of degradation by using citrate buffer instead of the usual 5 M hydrochloric acid to acidify the plasma. The method is also more specific and less time-consuming than the previously reported liquid-liquid extraction, and could possibly be automated.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
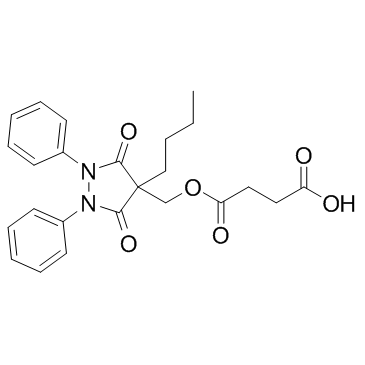 |
Suxibuzone
CAS:27470-51-5 |
C24H26N2O6 |
|
[The biological fate of 4-butyl-4-(beta-carboxypropionyloxym...
1979-12-01 [Yakugaku Zasshi 99(12) , 1186-200, (1979)] |
|
[The biological fate of 4-butyl-4-(beta-carboxypropionyloxym...
1980-03-01 [Yakugaku Zasshi 100(3) , 272-9, (1980)] |
|
Pro-drugs of non-steroidal anti-inflammatory agents.
1983-01-01 [Eur. J. Rheumatol. Inflamm. 6(2) , 141-2, (1983)] |
|
Antemortem diagnosis of a distal axonopathy causing severe s...
2010-01-01 [J. Vet. Intern. Med. 24(1) , 220-3, (2010)] |
|
Disposition of human drug preparations in the horse. I. Rect...
1991-06-01 [J. Vet. Pharmacol. Ther. 14(2) , 145-9, (1991)] |