15N and 13C NMR chemical shifts of 6-(fluoro, chloro, bromo, and iodo)purine 2'-deoxynucleosides: measurements and calculations.
Shi Bai, Olga Dmitrenko, Cecil Dybowski
Index: Magn. Reson. Chem. 48(1) , 61-7, (2010)
Full Text: HTML
Abstract
The (15)N and (13)C chemical shifts of 6-(fluoro, chloro, bromo, and iodo)purine 2'-deoxynucleoside derivatives in deuterated chloroform were measured. The (15)N chemical shifts were determined by the (1)H-(15)N HMBC method, and complete (15)N chemical-shift assignments were made with the aid of density functional theory (DFT) calculations. Inclusion of solvation effects significantly improved the precision of the calculations of (15)N chemical shifts. Halogen-substitution effects on the (15)N and (13)C chemical shifts of purine rings are discussed in the context of DFT results. The experimental coupling constants for (19)F interacting with (15)N and (13)C of the 6-fluoropurine 2-deoxynuleoside are compared with those from DFT calculations.Copyright 2009 John Wiley & Sons, Ltd.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
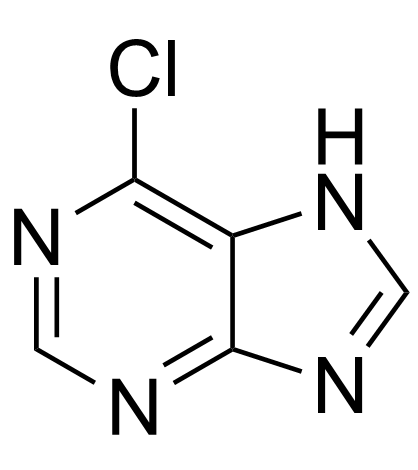 |
6-chloropurine
CAS:87-42-3 |
C5H3ClN4 |
|
Synthesis of modified homo-N-nucleosides from the reactions ...
2009-01-01 [Bioorg. Med. Chem. Lett. 19 , 6433-6, (2009)] |
|
The synthesis of novel fluorescent purine analogues modified...
2010-01-01 [Bioorg. Med. Chem. Lett. 20(10) , 3098-102, (2010)] |
|
Evidence for Watson-Crick and not Hoogsteen or wobble base p...
2005-03-29 [Biochemistry 44(12) , 4850-60, (2005)] |
|
Asymmetric synthesis of novel thioiso dideoxynucleosides wit...
2004-04-30 [J. Org. Chem. 69(9) , 3208-11, (2004)] |
|
Synthesis and biological evaluation of nucleoside analogues ...
2007-05-01 [Bioorg. Med. Chem. Lett. 17 , 2470-3, (2007)] |