| Structure | Name/CAS No. | Articles |
|---|---|---|
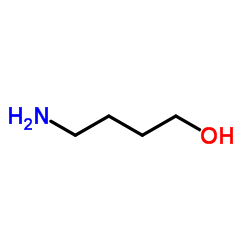 |
4-Amino-1-butanol
CAS:13325-10-5 |
|
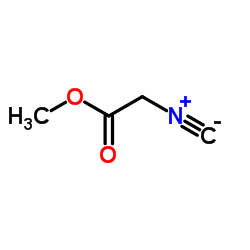 |
Methyl isocyanoacetate
CAS:39687-95-1 |