Determination of cardiac glycosides in digitoxin tablets and deslanoside injections by micro-HPLC.
Y Fujii, Y Ikeda, M Yamazaki
Index: J. Chromatogr. Sci. 28(6) , 288-91, (1990)
Full Text: HTML
Abstract
A micro high-performance liquid chromatographic (micro-HPLC) procedure for the assay of digitoxin tablets and deslanoside injections has been developed. Micro-HPLC is performed on an ODS micro column, with acetonitrile-methanol-water (10:20:17) for digitoxin tablets and acetonitrile-water (21:70) for deslanoside injections. The effluent is monitored by UV absorption at 220 nm. Quantitation of cardiac glycosides in tablets and injections is carried out by the internal standard method. The composite assay results for digitoxin tablets and deslanoside injections provide average values of 101.2 and 99.9% with standard deviations of 1.2 and 0.93%, respectively. This micro-HPLC method is sensitive, quantitative, and reproducible. It is suitable for use in examining the content uniformity of pharmaceutical preparations.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
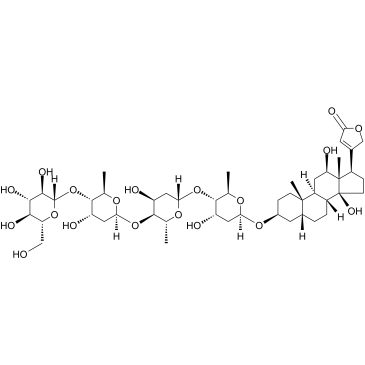 |
Deslanoside
CAS:17598-65-1 |
C47H74O19 |
|
Effects of acute administration of ethanol on experimental a...
2013-01-01 [Chin. J. Physiol. 55(5) , 307-13, (2012)] |
|
Altered vagal and sympathetic control of heart rate in left ...
1995-02-01 [Am. J. Physiol. 268(2 Pt 2) , R310-16, (1995)] |
|
Two-stage cultivation of Digitalis lanata cells: semicontinu...
1990-10-01 [J. Biotechnol. 16(1-2) , 123-35, (1990)] |
|
21'-Di-dehydro-deacetyllanatoside C, a biotransformation pro...
1997-03-01 [Phytochemistry 44(6) , 1061-4, (1997)] |
|
Clinical use of digitalis glycosides. An update.
1985-01-01 [Cardiology 72(5-6) , 225-54, (1985)] |