| Structure | Name/CAS No. | Articles |
|---|---|---|
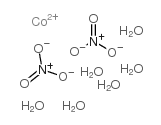 |
Cobaltous nitrate hexahydrate
CAS:10026-22-9 |
|
 |
Potassium 3-sulfotrioxidan-1-ide
CAS:70693-62-8 |
|
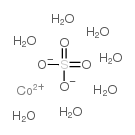 |
Cobalt sulfate heptahydrate
CAS:10026-24-1 |
|
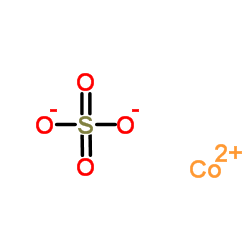 |
Cobalt sulfate
CAS:10124-43-3 |
|
 |
Cobalt powder
CAS:10141-05-6 |