Interaction of clozapine and its nitrenium ion with rat D2 dopamine receptors: in vitro binding and computational study.
Sébastien Dilly, Jean-François Liégeois
Index: J. Comput. Aided Mol. Des. 25(2) , 163-9, (2011)
Full Text: HTML
Abstract
The interaction of diazepine analogues like clozapine or olanzapine with D2 receptor was greatly affected by a mixture of HRP/H(2)O(2) known to induce the formation of nitrenium ion. Unlike diazepine derivatives, the oxidative mixture had low impact on the affinity of oxa- and thiazepine derivatives such as loxapine, clothiapine or JL13 for the D2 receptor. Molecular docking simulations revealed a huge difference between the mode of interaction of clozapine nitrenium ion and the parent drug. Electronic and geometric changes of the tricyclic ring system caused by the oxidation appeared to prevent the compound finding the correct binding mode and could therefore explain the difference observed in binding affinities.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
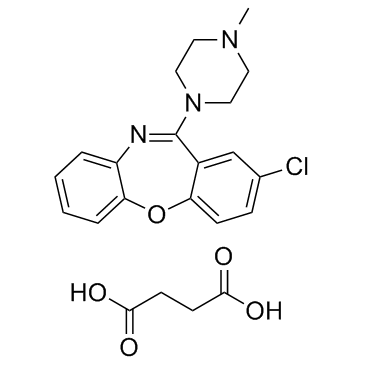 |
Loxapine succinate
CAS:27833-64-3 |
C22H24ClN3O5 |
|
Investigation of the disposition of loxapine, amoxapine and ...
2012-01-01 [J. Pharm. Biomed. Anal. 58 , 83-93, (2012)] |
|
In vitro aerosol characterization of Staccato(®) Loxapine.
2011-01-17 [Int. J. Pharm. 403(1-2) , 101-8, (2011)] |
|
Inhaled loxapine for agitation revisited: focus on effect si...
2012-03-01 [Int. J. Clin. Pract. 66(3) , 318-25, (2012)] |
|
The pharmacoepidemiology of antipsychotics for adults with s...
2011-10-01 [Can. J. Psychiatry. 56(10) , 630-4, (2011)] |
|
The antipsychotic drug loxapine is an opener of the sodium-a...
2012-03-01 [J. Pharmacol. Exp. Ther. 340(3) , 706-15, (2012)] |