| Structure | Name/CAS No. | Articles |
|---|---|---|
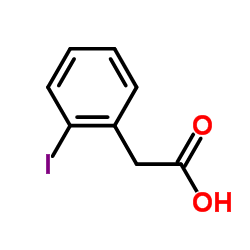 |
2-Iodophenylacetic acid
CAS:18698-96-9 |
|
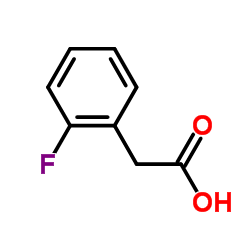 |
2-(2-Fluorophenyl)acetic acid
CAS:451-82-1 |