| Structure | Name/CAS No. | Articles |
|---|---|---|
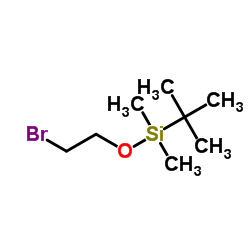 |
(2-Bromoethoxy)-Tert-Butyldimethylsilane
CAS:86864-60-0 |
| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
(2-Bromoethoxy)-Tert-Butyldimethylsilane
CAS:86864-60-0 |