| Structure | Name/CAS No. | Articles |
|---|---|---|
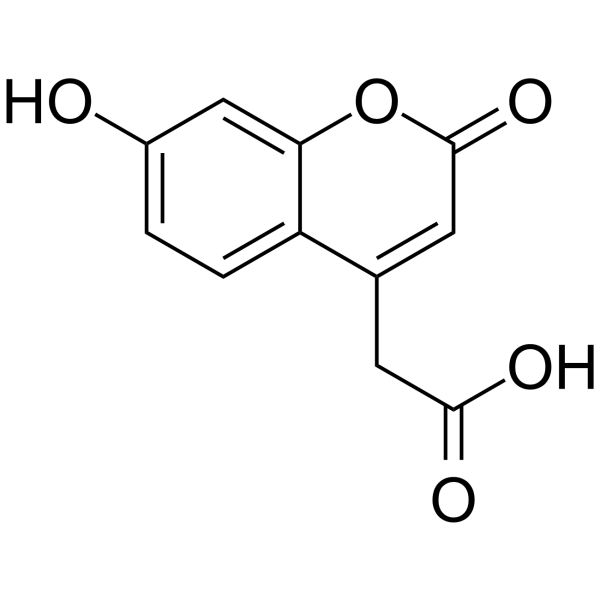 |
2H-1-Benzopyran-4-aceticacid, 7-hydroxy-2-oxo
CAS:6950-82-9 |
|
 |
Genistein
CAS:446-72-0 |
|
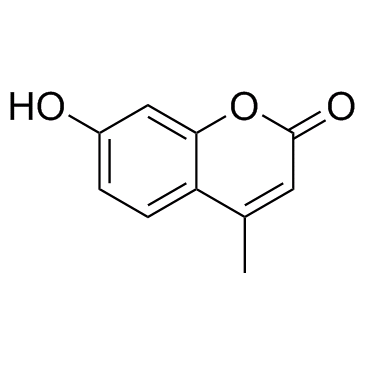 |
4-Methylumbelliferone
CAS:90-33-5 |
|
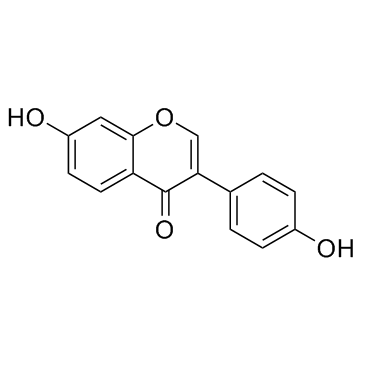 |
Daidzein
CAS:486-66-8 |
|
 |
Kaempferol
CAS:520-18-3 |