Labelling of recombinant human and native rat serotonin 5-HT1A receptors by a novel, selective radioligand, [3H]-S 15535: definition of its binding profile using agonists, antagonists and inverse agonists.
A Newman-Tancredi, L Verrièle, C Chaput, M J Millan
Index: Naunyn Schmiedebergs Arch. Pharmacol. 357(3) , 205-17, (1998)
Full Text: HTML
Abstract
The novel benzodioxopiperazine, 5-HT1A receptor weak partial agonist, S 15535 (4-(benzodioxan-5-yl)1-(indan-2-yl)piperazine) bound with high affinity and selectivity to membranes of Chinese Hamster Ovary cells stably expressing the human (h) 5-HT1A receptor (Ki = 0.6 nM versus [3H]-8-hydroxy-dipropylamino-tetralin, [3H]-8-OH-DPAT): its affinity at h5-HT1A receptors was more than 70-fold higher than its affinity at > 50 other binding sites. S 15535 was tritiated to high specific activity (50 Ci/mmol) and its binding profile characterised. At 22 degrees C, [3H]-S 15535 associated and dissociated from h5-HT1A receptors with half-times of 2.9 and 5.0 min, respectively, yielding a Kd estimate of 3.6 nM. In saturation binding experiments, [3H]-S 15535 displayed a Bmax value for h5-HT1A receptors (1630 fmol/mg), higher than that obtained with the agonist [3H]-8-OH-DPAT (1023 pmol/mg). Guanylyl imidodiphosphate (GppNHp, 100 microM) reduced the binding of [3H]-S 15535 by only 25% compared with 79% for [3H]-8-OH-DPAT at h5-HT1A receptors. [3H]-S 15535 also showed high affinity, saturable binding to rat hippocampal membranes (Bmax = 820 fmol/mg versus 647 fmol/mg for [3H]-8-OH-DPAT). For both h5-HT1A and rat 5-HT1A receptors, the Ki values for competition binding of 15 serotonergic ligands with [3H]-S 15535 was highly correlated with that of [3H]-8-OH-DPAT. However, important differences were also observed. The agonist, 5-hydroxytryptamine (5-HT), displayed biphasic competition curves with [3H]-S 15535 but not with [3H]-8-OH-DPAT at h5-HT1A receptors. Similarly, the 'antagonists', spiperone, methiothepin and (+)butaclamol, showed biphasic competition isotherms versus [3H]-S 15535 but not [3H]-8-OH-DPAT. When [3H]-S 15535 competition binding experiments were carried out in the presence of GppNHp (100 microM) the 5-HT and 8-OH-DPAT competition curves shifted to the right, whereas the spiperone and methiothepin competition curves shifted to the left. In contrast, in the presence of GppNHp, the competition isotherms for N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridinyl)cyclo-h exanecarboxamide (WAY 100,635) were not altered. Taken together, these data show that (i) [3H]-S 15535 is a highly selective 5-HT1A receptor ligand which labels both G-protein-coupled and uncoupled 5-HT1A receptors, (ii) antagonists, such as WAY 100,635, which yield monophasic isotherms in competition with both [3H]-agonists and [3H]-antagonists, are not sensitive to the G-protein coupling state of the receptor, but (iii) spiperone and methiothepin behaved as inverse agonists, their competition isotherms with [3H]-S 15535 being modulated in an opposite manner to those of agonists.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
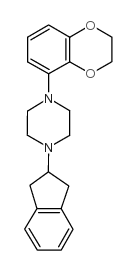 |
S-15535
CAS:146998-34-7 |
C21H24N2O2 |
|
S 15535, a novel benzodioxopiperazine ligand of serotonin (5...
1997-07-01 [J. Pharmacol. Exp. Ther. 282(1) , 132-47, (1997)] |
|
Differential modulation by GTPgammaS of agonist and inverse ...
2001-01-01 [Br. J. Pharmacol. 132(2) , 518-24, (2001)] |
|
Improvement in the selectivity and metabolic stability of th...
2002-01-03 [J. Med. Chem. 45(1) , 165-76, (2002)] |
|
S 15535 and WAY 100,635 antagonise 5-HT-stimulated [35S]GTP ...
1996-06-20 [Eur. J. Pharmacol. 307(1) , 107-11, (1996)] |
|
Serotonin1A autoreceptor activation by S 15535 enhances circ...
2006-01-01 [Neuroscience 137(1) , 287-99, (2006)] |