Chiral high-performance liquid chromatography of N-octyl bicycloheptene dicarboximide and confirmatory studies using liquid chromatography-tandem mass spectrometry and two-dimensional nuclear magnetic resonance spectroscopy.
I H Wang, V Subramanian, R Moorman, J Burleson, J Ko
Index: J. Chromatogr. A. 864(2) , 271-81, (1999)
Full Text: HTML
Abstract
N-Octyl bicycloheptene dicarboximide (MGK 264) has exo and endo diastereomers. Each structure has a chiral center at the nitrogen side chain. Enantioselective separation of MGK 264 was achieved by normal-phase high-performance liquid chromatography (HPLC) using cellulose-based Chiralcel OD column with diode-array and optical rotation detectors. Peaks were isolated with the purpose of identifying their stereochemical structures. Molecular mass of the HPLC peaks and their structural information was determined by liquid chromatography-electrospray tandem mass spectrometry (LC-ES-MS-MS). A two-dimensional nuclear magnetic resonance (NMR) spectroscopic technique was used to establish the structural features. Correlation of the data obtained from chiral separation and NMR facilitated in unambiguous assignment of the HPLC peaks.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
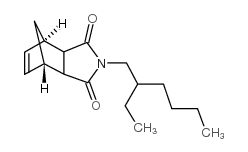 |
MGK-264
CAS:113-48-4 |
C17H25NO2 |
|
Effects of synergists on bendiocarb and pyrethrins resistanc...
1987-08-01 [J. Econ. Entomol. 80(4) , 728-32, (1987)] |
|
Effect of binary combination of some plant-derived molluscic...
2005-02-01 [Pest Manag. Sci. 61(2) , 204-8, (2005)] |
|
Isocratic reversed-phase liquid chromatographic method for t...
2003-01-03 [J. Chromatogr. A. 983(1-2) , 145-52, (2003)] |
|
Pathological laughter.
1990-03-01 [Ann. Emerg. Med. 19(3) , 327-9, (1990)] |
|
Effect of different combinations of MGK-264 or piperonyl but...
2000-02-01 [Arch. Environ. Contam. Toxicol. 38(2) , 182-90, (2000)] |