Bioelectrochemistry
2010-02-01
Electrochemical behaviour of dimethyl-2-oxoglutarate on glassy carbon electrode.
Afzal Shah, Victor C Diculescu, Rumana Qureshi, Ana Maria Oliveira-Brett
Index: Bioelectrochemistry 77(2) , 145-50, (2010)
Full Text: HTML
Abstract
The electrochemical behaviour of dimethyl-2-oxoglutarate (MOG), a key intermediate in the Krebs cycle and an important nitrogen transporter in the metabolic pathways in biological processes, was investigated by cyclic voltammetry, square wave voltammetry and differential pulse voltammetry using a glassy carbon electrode. The reduction of MOG is an irreversible diffusion-controlled process that occurs in a cascade mechanism. For electrolytes with pH <3.0 and pH >7.0 one peak occurred and for 3.0
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
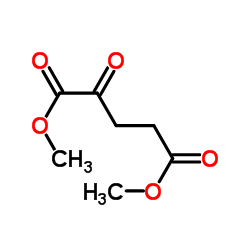 |
Dimethyl 2-oxopentanedioate
CAS:13192-04-6 |
C7H10O5 |
Related Articles:
More...
|
Deficiencies in Cellular Processes Modulated by the Retinobl...
2015-12-01 [J. Virol. 89 , 11965-74, (2015)] |
|
Mesenchymal phenotype predisposes lung cancer cells to impai...
2014-01-01 [PLoS ONE 9(12) , e115144, (2014)] |
|
Glutamine Regulates Cardiac Progenitor Cell Metabolism and P...
2015-08-01 [Stem Cells 33 , 2613-27, (2015)] |
|
A novel PNA-monomer for recognition of thymine in triple-hel...
2003-01-01 [Nucleosides Nucleotides Nucleic Acids 22(5-8) , 1331-3, (2003)] |
|
Intermediary metabolite precursor dimethyl-2-ketoglutarate s...
2014-01-01 [PLoS ONE 9(11) , e113865, (2014)] |