Effects of phenol compounds, glutathione analogues and a diuretic drug on glutathione S-transferase, glutathione reductase and glutathione peroxidase from canine erythrocytes.
M Kurata, M Suzuki, K Takeda
Index: Comp. Biochem. Physiol.,. B. 103(4) , 863-7, (1992)
Full Text: HTML
Abstract
1. Phenol compounds (ellagic acid, quercetin and purpurogallin), glutathione analogues (S-hexylglutathione and S-octylglutathione) and a diuretic drug (ethacrynic acid) were compared for their inhibitory effects on glutathione S-transferase (GST), glutathione reductase (GR) and glutathione peroxidase (GSH-Px) in the canine erythrocytes. 2. All these compounds inhibited GST activity; quercetin was found to be the most potent inhibitor. 3. Ellagic acid, purpurogallin, quercetin and ethacrynic acid inhibited GR activity; S-hexylglutathione and S-octylglutathione had no effect on GR and GSH-Px activities. 4. Quercetin and purpurogallin inhibited GST non-competitively toward glutathione, whereas ellagic acid showed a competitive inhibition. Ellagic acid and purpurogallin inhibited GR non-competitively toward oxidized glutathione.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
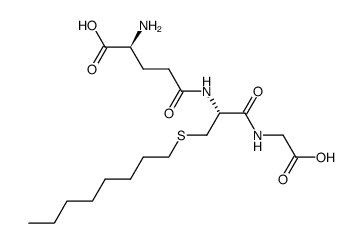 |
S-Octylglutathione
CAS:24435-27-6 |
C18H33N3O6S |
|
Crystallographic and thermodynamic analysis of the binding o...
2005-02-01 [Biochemistry 44 , 1174-1183, (2005)] |
|
An XAS investigation of product and inhibitor complexes of N...
2001-04-17 [Biochemistry 40(15) , 4569-82, (2001)] |
|
A calorimetric study of the binding of S-alkylglutathiones t...
2001-07-09 [Biochim. Biophys. Acta 1548(1) , 106-13, (2001)] |
|
Role of a partially purified glutathione S-transferase from ...
1989-04-06 [Biochim. Biophys. Acta 995(2) , 174-80, (1989)] |
|
Characterization of leukotriene C4 binding in anterior pitui...
1991-02-01 [Prostaglandins 41(2) , 185-99, (1991)] |