Solid-phase synthesis of tailed cyclic RGD peptides using glutamic acid: unexpected glutarimide formation.
Junmin Zhu, Roger E Marchant
Index: J. Pept. Sci. 14 , 690-696, (2008)
Full Text: HTML
Abstract
To provide multiple conjugating sites on cyclic peptides for their increasing biomedical applications, a tailed cyclic RGD peptide, c[RGDfE(GGGKK-NH(2))] was designed with c(RGDfE) linked through Glu to a tail consisting of a spacer of three Gly residues and a linker of two Lys residues. The spacer is used to increase the mobility and binding ability of the c(RGDfE) ligand, and the linker is used to proved multiple active sites for conjugating other molecules or biomaterials. We found that the sequence of Glu(Gly)-OAll leads to glutarimide formation, which disrupts the formation of cyclic RGD peptides. However, our results show that glutarimide formation is sequence dependent and can be inhibited by incorporating an amino acid like Lys(Boc) with steric hindrance from the protecting group. To prevent glutarimide formation, Ser(tBu) was used to replace the glycine in the GGG spacer adjacent to the residue of Glu, and a tailed cyclic RGD peptide, c[RGDfE(SGGKK-NH(2))] was successfully obtained.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
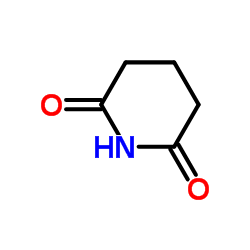 |
Glutarimide
CAS:1121-89-7 |
C5H7NO2 |
|
Comparative characterization of the lactimidomycin and iso-m...
2014-12-16 [Biochemistry 53(49) , 7854-65, (2014)] |
|
Iso-migrastatin congeners from Streptomyces platensis and ge...
2005-08-31 [J. Am. Chem. Soc. 127(34) , 11930-1, (2005)] |
|
Enantiomerization mechanism of thalidomide and the role of w...
2012-11-05 [Chemistry 18(45) , 14305-13, (2012)] |
|
Evaluation of new migrastatin and dorrigocin congeners unvei...
2008-11-15 [Bioorg. Med. Chem. Lett. 18(22) , 5951-4, (2008)] |
|
Inhibition of macrophage activation and suppression of graft...
2011-09-01 [Inflamm. Res. 60(9) , 879-88, (2011)] |