A quantitative NMR protocol for the simultaneous analysis of atropine and obidoxime in parenteral injection devices.
Radha Sharma, Pradeep K Gupta, Avik Mazumder, Devendra K Dubey, Kumaran Ganesan, R Vijayaraghavan
Index: J. Pharm. Biomed. Anal. 49(4) , 1092-6, (2009)
Full Text: HTML
Abstract
A rapid selective and accurate quantitative (1)H NMR method was developed for the simultaneous analysis of obidoxime chloride and atropine sulfate, the active components in parenteral injection devices (PID) used for the emergency treatment of poisoning by toxic organophosphates. The spectra were acquired in 90% H(2)O-10% D(2)O using sodium 3-(trimethylsilyl)-1-propane sulfonate hydrate as the internal standard. Both synthetic mixtures and dosage forms were assayed. The results were compared with those obtained from a reported HPLC method.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
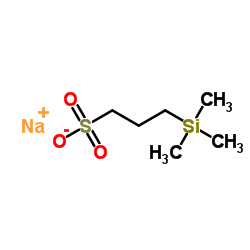 |
sodium trimethylsilylpropylsulfonate
CAS:2039-96-5 |
C6H15NaO3SSi |
|
1H-NMR-based profiling of organic components in leachate fro...
2014-09-01 [Environ. Sci. Pollut. Res. Int. 21(17) , 10453-60, (2014)] |
|
Structural elucidation of an asparagine-linked oligosacchari...
2015-09-02 [Carbohydr. Res. 413 , 55-62, (2015)] |
|
Shuttle DNP spectrometer with a two-center magnet.
2010-06-14 [Phys. Chem. Chem. Phys. 12(22) , 5830-40, (2010)] |
|
Synthesis of DOTA-conjugated multivalent cyclic-RGD peptide ...
2007-03-21 [Org. Biomol. Chem. 5(6) , 935-44, (2007)] |
|
Chemoenzymatic synthesis and structural characterization of ...
2014-08-01 [Glycobiology 24(8) , 681-92, (2014)] |