Novel pathways of oleic and cis-vaccenic acid biosynthesis by an enzymatic double-bond shifting reaction in higher plants.
A Shibahara, K Yamamoto, M Takeoka, A Kinoshita, G Kajimoto, T Nakayama, M Noda
Index: FEBS Lett. 264(2) , 228-30, (1990)
Full Text: HTML
Abstract
The novel pathways of oleic acid formation from cis-vaccenic (cis-11-octadecenoic) acid and of cis-vaccenic acid formation from oleic acid by enzymatic positional isomerization have been proposed in higher plants, based on stable-isotope experiments using [2,2-2H2]cis-vaccenate or [2,2-2H2]oleate as an immediate precursor. A pulp homogenate and also pulp slices prepared from developing kaki (Diospyros kaki) fruit could catalyze these hitherto unknown isomerizations. This suggests the presence of a new type of isomerase responsible for the double-bond shifting reaction without cis-trans isomerization in the middle of fatty acid carbon chains.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
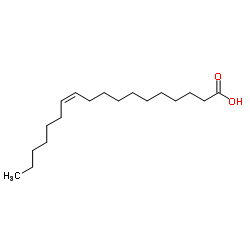 |
cis-Vaccenic acid
CAS:506-17-2 |
C18H34O2 |
|
Characterization of Shrimp Oil from Pandalus borealis by Hig...
2015-06-01 [Mar. Drugs 13 , 3849-76, (2015)] |
|
Palmitoleic (16:1 cis-9) and cis-vaccenic (18:1 cis-11) acid...
2012-12-01 [Lipids 47(12) , 1143-53, (2012)] |
|
Red blood cell membrane concentration of cis-palmitoleic and...
2012-08-15 [Am. J. Cardiol. 110(4) , 539-44, (2012)] |
|
Effects of dietary cis 9, trans 11-18:2, trans 10, cis 12-18...
2003-12-01 [Br. J. Nutr. 90(6) , 1039-48, (2003)] |
|
Replacing cis octadecenoic acid with trans isomers in media ...
1995-09-01 [J. Nutr. 125(9) , 2394-9, (1995)] |