| Structure | Name/CAS No. | Articles |
|---|---|---|
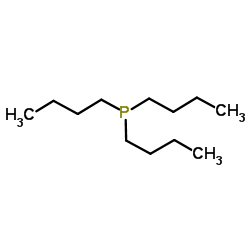 |
Tributylphosphine
CAS:998-40-3 |
|
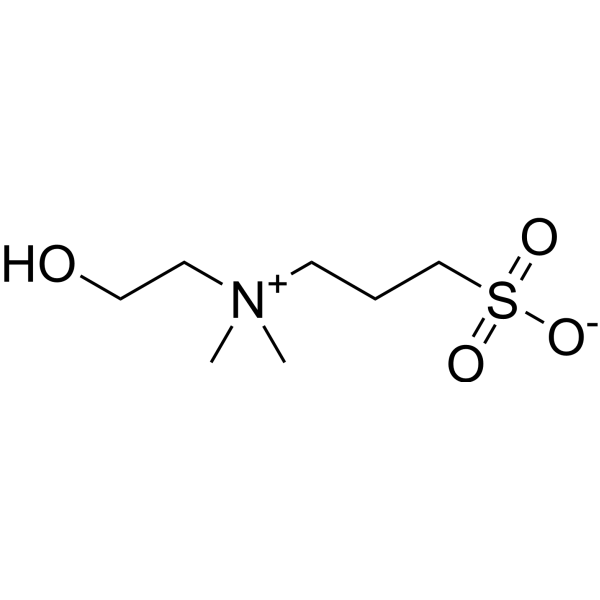 |
NDSB-211
CAS:38880-58-9 |