Review of developmental toxicity of nitrophenolic herbicide dinoseb, 2-sec-butyl-4,6-dinitrophenol.
Mariko Matsumoto, Carlo Poncipe, Makoto Ema
Index: Reprod. Toxicol. 25(3) , 327-34, (2008)
Full Text: HTML
Abstract
The present review paper summarizes the data available in the literature concerning prenatal exposure to dinoseb (2-sec-butyl-4,6-dinitrophenol; CAS No. 88-85-7), evaluating reported developmental toxicity in experimental animals. In particular, we have focused on the variable factors in the manifestation of the developmental toxicity of dinoseb. In this review, we showed that developmental toxicity of dinoseb was remarkably different between animal species used in experiments. Teratogenicity was detected in rats fed a diet containing dinoseb, in mice given dinoseb by gavage, intraperitoneally or subcutaneously, and in rabbits given dinoseb by gavage or dermally. Teratogenicity in rats given dinoseb by gavage was influenced by the dietary composition used in the experiments. We postulated that evaluation of the developmental toxicity after exposure by anticipated routes of human exposure would be important for risk assessment in humans.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
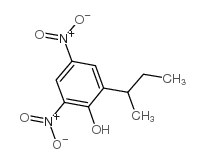 |
dinoseb
CAS:88-85-7 |
C10H12N2O5 |
|
Combined repeated dose and reproductive/developmental toxici...
2008-04-01 [Environ. Toxicol. 23(2) , 169-83, (2008)] |
|
Comparative studies on the spermatotoxic effects of dinoseb ...
2004-06-01 [Reprod. Toxicol. 18(4) , 581-8, (2004)] |
|
Simultaneous determination of three herbicides by differenti...
2011-01-01 [J. Environ. Sci. Health B 46(4) , 328-35, (2011)] |
|
Photoassisted degradation of a herbicide derivative, dinoseb...
2012-01-01 [ScientificWorldJournal 2012 , 251527, (2012)] |
|
Effects of dinoseb, 4,6-dinitro-o-cresol, and 2,4-dinitrophe...
2003-01-01 [Reprod. Toxicol. 17(2) , 247-52, (2003)] |