| Structure | Name/CAS No. | Articles |
|---|---|---|
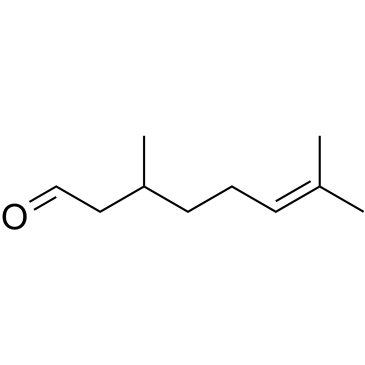 |
Citronellal
CAS:106-23-0 |
|
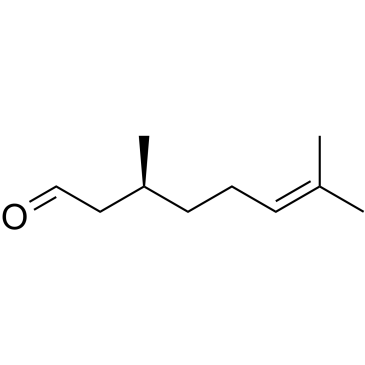 |
(-)-citronellal
CAS:5949-05-3 |
|
 |
(+)-Citronellal
CAS:2385-77-5 |