| Structure | Name/CAS No. | Articles |
|---|---|---|
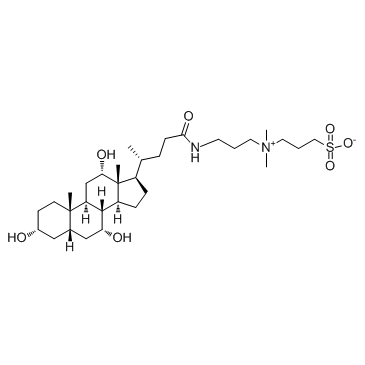 |
CHAPS
CAS:75621-03-3 |
|
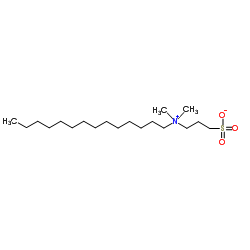 |
3-(N,N-Dimethylmyristylammonio)propanesulfonate
CAS:14933-09-6 |