| Structure | Name/CAS No. | Articles |
|---|---|---|
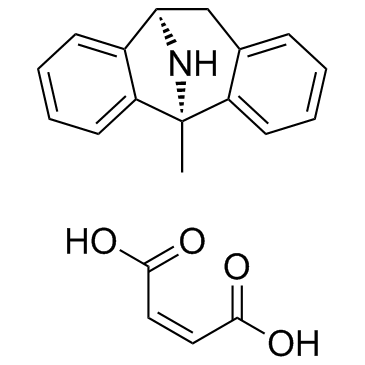 |
(+)-MK 801 Maleate
CAS:77086-22-7 |
|
 |
D-Amino acid oxidase
CAS:9000-88-8 |