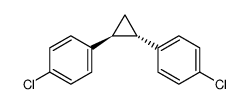
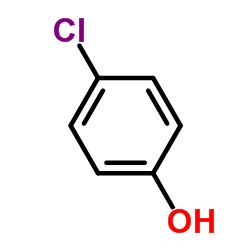
|
~36% |
|
~73% |
|
~% |
|
~6% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~41% |
|
~45% |
|
~% |
|
~% |
|
~% |
|
~87% |
|
~% |
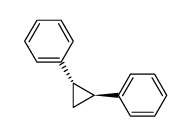
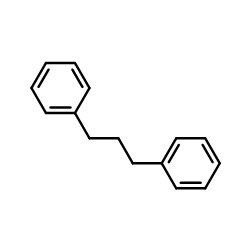
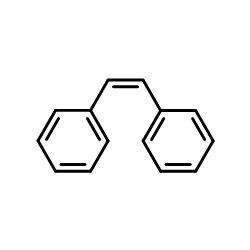
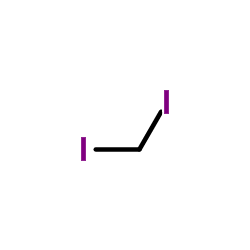
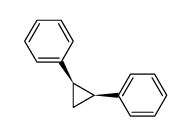


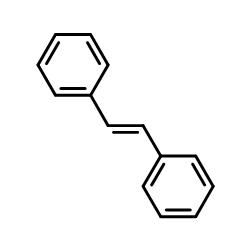
![1-chloro-4-[(E)-2-(4-chlorophenyl)vinyl]benzene Structure](https://image.chemsrc.com/caspic/340/2510-74-9.png)