Bioorganic & Medicinal Chemistry Letters
2008-11-15
A highly sensitive fluorogenic probe for cytochrome P450 activity in live cells.
Melissa M Yatzeck, Luke D Lavis, Tzu-Yuan Chao, Sunil S Chandran, Ronald T Raines
Index: Bioorg. Med. Chem. Lett. 18(22) , 5864-6, (2008)
Full Text: HTML
Abstract
A derivative of rhodamine 110 has been designed and assessed as a probe for cytochrome P450 activity. This probe is the first to utilize a 'trimethyl lock' that is triggered by cleavage of an ether bond. In vitro, fluorescence was manifested by the CYP1A1 isozyme with k(cat)/K(M)=8.8x10(3)M(-1)s(-1) and K(M)=0.09microM. In cellulo, the probe revealed the induction of cytochrome P450 activity by the carcinogen 2,3,7,8-tetrachlorodibenzo-p-dioxin, and its repression by the chemoprotectant resveratrol.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
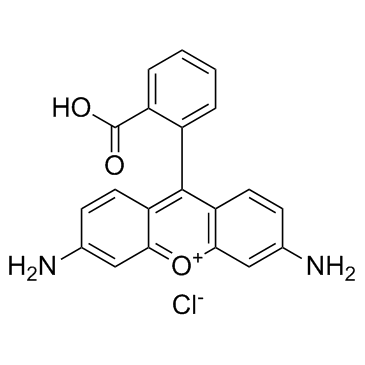 |
Rhodamine 110
CAS:13558-31-1 |
C20H15ClN2O3 |
Related Articles:
More...
|
Rhodamine-based fluorogenic probe for imaging biological thi...
2008-04-01 [Bioorg. Med. Chem. Lett. 18(7) , 2246-9, (2008)] |
|
Early detection of apoptosis in living cells by fluorescence...
2010-02-01 [Anal. Bioanal. Chem 396(3) , 1177-85, (2010)] |
|
Mapping vortex-like hydrodynamic flow in microfluidic networ...
2009-09-28 [Anal. Chim. Acta 651(1) , 85-90, (2009)] |
|
Effects of polyoxyethylene (40) stearate on the activity of ...
2009-07-12 [Eur. J. Pharm. Sci. 37(5) , 573-80, (2009)] |
|
Rhamnolipids enhance epithelial permeability in Caco-2 monol...
2013-03-25 [Int. J. Pharm. 446(1-2) , 130-5, (2013)] |