| Structure | Name/CAS No. | Articles |
|---|---|---|
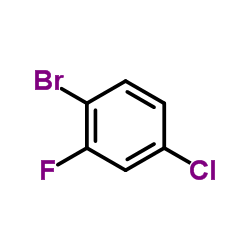 |
1-Bromo-4-chloro-2-fluorobenzene
CAS:1996-29-8 |
|
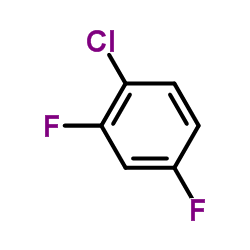 |
1-Chloro-2,4-difluorobenzene
CAS:1435-44-5 |