[Partial characteristics of the basic phenylmethylsulfonylfluoride-inhibited carboxypeptidase from cat brain].
A N Vernigora, N N Nikishin, M T Gengin
Index: Biokhimiia 60(11) , 1860-6, (1995)
Full Text: HTML
Abstract
Cat brain carboxypeptidase releasing C-terminal arginine from the synthetic substrate, dansyl-Phe-Leu-Arg, was partially characterized. The enzyme has a molecular weight of 100-120 kDa, displays the maximal activity at pH 6.0-6.5 and is strongly inhibited by phenylmethanesulfonylfluoride and 4-chloromercuribenzoate, less strongly (by 40%) by iodoacetamide and is not inhibited by N-ethylmaleimide, 2-mercaptoethanol, EDTA, Co2+ and guanidinoethylmercaptosuccinic acid. The Km values for the hydrolysis of dansyl-Phe-Leu-Arg and dansyl-Phe-Ala-Arg by soluble carboxypeptidase are 48 and 96 microM, respectively. By all properties, this carboxypeptidase differs from other known carboxypeptidases. Possible participation of the enzyme in neuropeptide metabolism is discussed.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
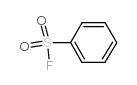 |
Benzenesulfonylfluoride
CAS:368-43-4 |
C6H5FO2S |
|
Low concentrations of Bisphenol A and para-Nonylphenol affec...
2015-09-05 [Mol. Cell. Endocrinol. 412 , 56-64, (2015)] |
|
Downregulation of the expression of B‑cell lymphoma-extra la...
2015-07-01 [Mol. Med. Report. 12 , 449-55, (2015)] |
|
Identification of in vivo-induced bacterial protein antigens...
2015-05-01 [Int. J. Med. Microbiol. 305 , 310-21, (2015)] |
|
Alterations in the oxidative metabolism of Rhipicephalus (Bo...
2016-01-01 [Rev. Bras. Parasitol. Vet. 25 , 54-60, (2016)] |
|
Hydrogen sulfide alleviates cardiac contractile dysfunction ...
2014-05-15 [Am. J. Physiol. Regul. Integr. Comp. Physiol. 306(10) , R761-71, (2014)] |