| Structure | Name/CAS No. | Articles |
|---|---|---|
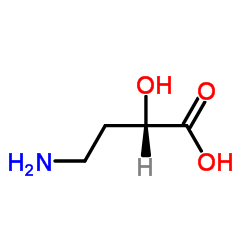 |
(S)-4-Amino-2-hydroxybutanoic acid
CAS:40371-51-5 |
| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
(S)-4-Amino-2-hydroxybutanoic acid
CAS:40371-51-5 |