Absorption and elimination of (14C) hesperidin methylchalcone in the rat.
J L Chanal, H Cousse, M T Sicart, B Bonnaud, R Marignan
Index: Eur. J. Drug Metab. Pharmacokinet. 6(3) , 171-7, (1981)
Full Text: HTML
Abstract
Hesperidin methylchalcone resorption and excretion were studied in rats, using 14C-labelling. The level of radioactivity in the blood showed a peak 1-2 hours after oral administration of the labelled compound, at a dose of 10 mg/kg body weight. The blood kinetics pattern suggested an entero-hepatic cycle, which was demonstrated by i.v. administration of the compound at the same dose. The blood profiles for both administration routes, demonstrated that the bioavailability of the active principle was good. Urinary excretion was lower than faecal excretion after oral ingestion, and both were comparable after administration via the i.v. route. Moreover, excretion mainly occurred within the first 24 hours following administration. When hesperidin methylchalcone was given in a therapeutic, pharmaceutical formulation, its bioavailability was greatly improved. (This was not due to the alcoholic ingredient in the formula).
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
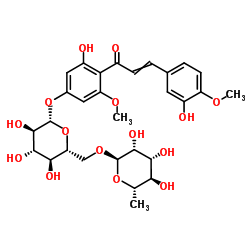 |
Hesperidin methylchalcone
CAS:24292-52-2 |
C29H36O15 |
|
Assessment of the antineoplastic potential of chalcones in a...
2013-01-01 [Curr. Med. Chem. 20(2) , 186-221, (2013)] |
|
Inhibitory effect of the Ruscus extract and of the flavonoid...
1993-08-01 [J. Cardiovasc. Pharmacol. 22(2) , 225-30, (1993)] |
|
Hesperidin, hesperidin methyl chalone and phellopterin from ...
2008-05-01 [Int. Immunopharmacol. 8(5) , 670-8, (2008)] |
|
Effect of Ruscus extract and hesperidin methylchalcone on hy...
1999-12-01 [Int. Angiol. 18(4) , 306-12, (1999)] |
|
Clinical and capillaroscopic evaluation in the treatment of ...
2007-12-01 [Int. Angiol. 26(4) , 378-84, (2007)] |