Carbohydrate Research
2006-07-24
De novo asymmetric syntheses of C-4-substituted sugars via an iterative dihydroxylation strategy.
Md Moinuddin Ahmed, George A O'Doherty
Index: Carbohydr. Res. 341(10) , 1505-21, (2006)
Full Text: HTML
Abstract
A short and highly efficient route to various C-4 substituted sugar lactones has been developed. The key to the overall transformation is the sequential osmium-catalyzed dihydroxylation reaction of substituted 2,4-dienoates and an allylic substitution at the C-4 position. When the Sharpless AD-mix procedure is used in a matched sense for the second dihydroxylation reaction, it results in an exceedingly diastereo- and enantioselective synthesis of several C-4-substituted sugars.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
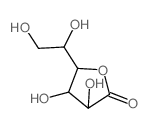 |
L-galactono-1,4-lactone
CAS:1668-08-2 |
C6H10O6 |
Related Articles:
More...
|
The biosynthesis of erythroascorbate in Saccharomyces cerevi...
2000-01-15 [Free Radic. Biol. Med. 28(2) , 183-92, (2000)] |
|
Cellular ascorbic acid regulates the activity of major perox...
2007-01-01 [Plant Physiol. Biochem. 45(3-4) , 188-98, (2007)] |
|
Galactone-γ-lactone-dependent ascorbate biosynthesis alters ...
2012-07-01 [Plant Biol. (Stuttg.) 14(4) , 652-8, (2012)] |
|
Hydroponically cultivated radish fed L-galactono-1,4-lactone...
2002-01-01 [Planta 214(3) , 383-91, (2002)] |
|
Expression of aspartyl protease and C3HC4-type RING zinc fin...
2011-06-01 [J. Exp. Bot. 62(10) , 3647-57, (2011)] |