Chemical dehydration of specimens with 2,2-dimethoxypropane (DMP) for paraffin processing of animal tissues: practical and economic advantages over dehydration in ethanol.
K Conway, J A Kiernan
Index: Biotech. Histochem. 74(1) , 20-6, (1999)
Full Text: HTML
Abstract
Chemical dehydration can be accomplished using 2,2-dimethoxypropane (DMP). In the presence of an acid catalyst, this liquid reacts with water generating methanol and acetone as products. Although DMP is more expensive per milliliter than ethanol and other solvents used for dehydration, it is an economical alternative because a much smaller volume is needed. Slow penetration of DMP was previously thought to restrict its use to tiny specimens, but we now show that pieces of tissue as thick as 2 cm are dehydrated by overnight immersion in acidified DMP. We also show that dehydration in acidified DMP does not impair the staining of RNA or other basophilic components of animal tissues. The temperature and concentrations of methanol and H+ in the chemical dehydrating agent are too low to produce histochemically detectable methylation or nucleic acid extraction.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
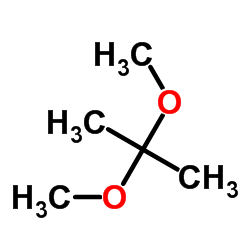 |
2,2-Dimethoxypropane
CAS:77-76-9 |
C5H12O2 |
|
Delivery of therapeutic protein for prevention of neurodegen...
2015-01-01 [Exp. Neurol. 263 , 79-90, (2014)] |
|
Preparation and synthetic application of partially protected...
2010-01-01 [Steroids 75(1) , 27-33, (2010)] |
|
Acetonation of L-pentoses and 6-deoxy-L-hexoses under kineti...
2010-07-19 [Carbohydr. Res. 345(11) , 1548-54, (2010)] |
|
Chemical dehydration for rapid paraffin embedding.
1994-09-01 [Biotech. Histochem. 69(5) , 289-90, (1994)] |
|
Direct transesterification of lipids in mammalian tissue for...
1977-07-01 [J. Lipid Res. 18(4) , 540-3, (1977)] |