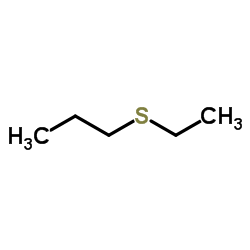
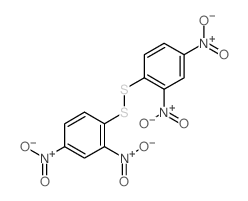
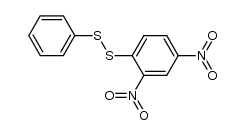
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
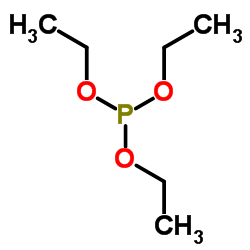
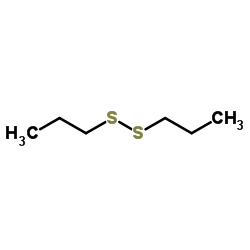
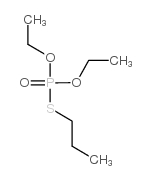
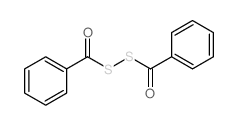
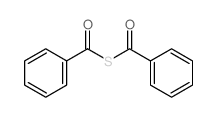
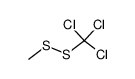
![1-[ethoxy(methylsulfanyl)phosphoryl]oxyethane Structure](https://image.chemsrc.com/caspic/148/2404-05-9.png)