| Structure | Name/CAS No. | Articles |
|---|---|---|
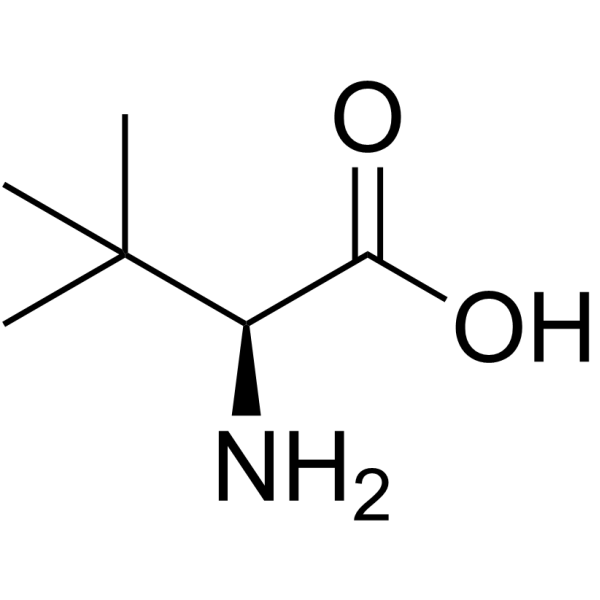 |
L-tert-Leucine
CAS:20859-02-3 |
|
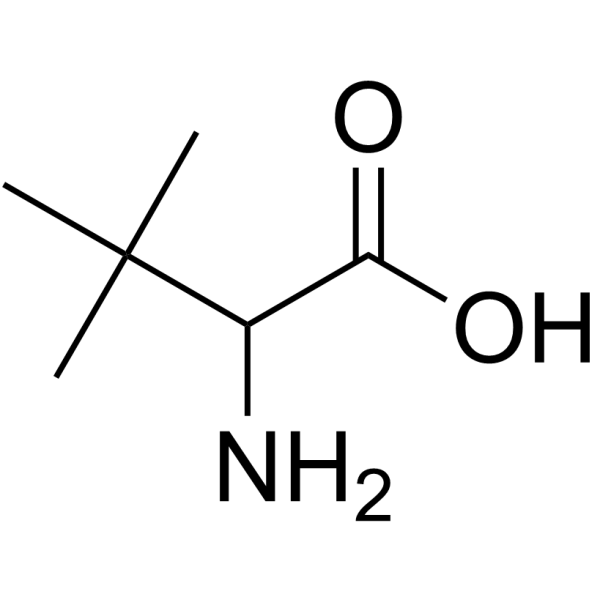 |
3-Methylvaline
CAS:33105-81-6 |