| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Sephadex G 75
CAS:37224-29-6 |
|
![[5-O-Phosphono-κ2O,O'pentofuranosato(2-)]barium Structure](https://image.chemsrc.com/caspic/475/15673-79-7.png) |
[5-O-Phosphono-κ2O,O'pentofuranosato(2-)]barium
CAS:15673-79-7 |
|
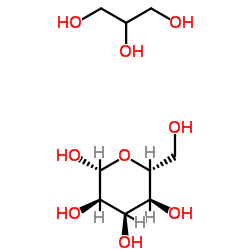 |
1,2,3-Propanetriol-β-D-allopyranose (1:1)
CAS:9041-35-4 |
|
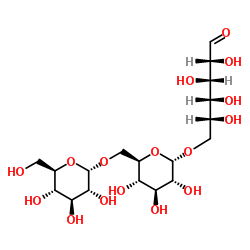 |
Dextran
CAS:9004-54-0 |
|
 |
SEPHADEX G-100
CAS:9050-94-6 |
|
 |
Sephadex G 50
CAS:9048-71-9 |
|
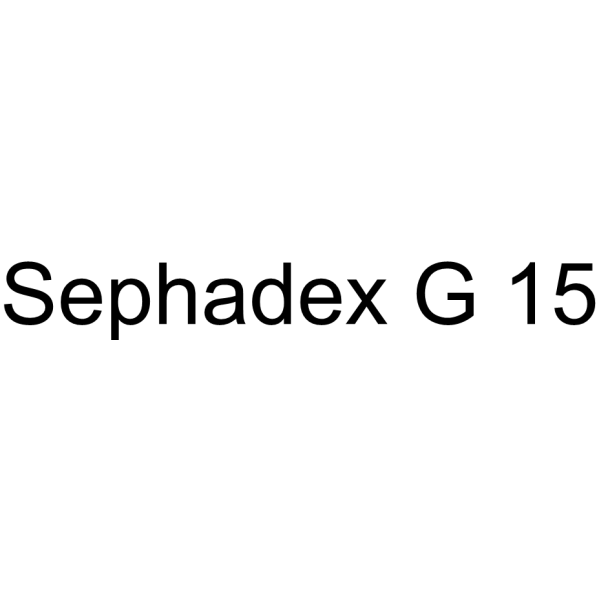 |
Sephadex G15
CAS:11081-40-6 |
|
 |
Sephadex G10
CAS:9050-68-4 |