| Structure | Name/CAS No. | Articles |
|---|---|---|
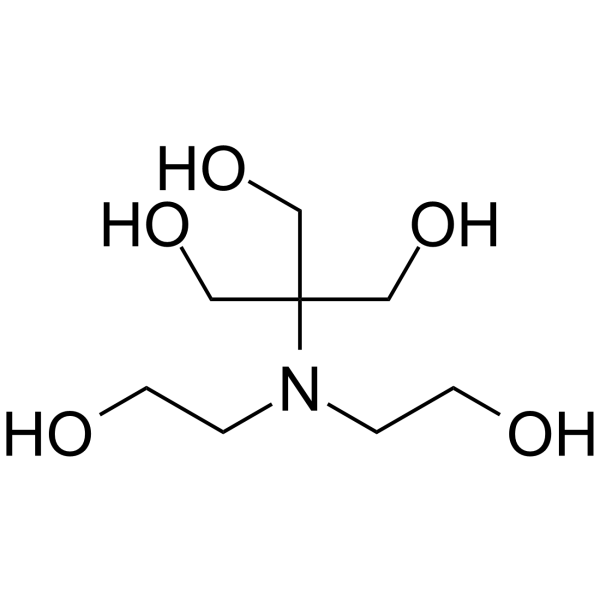 |
Bis-tris methane
CAS:6976-37-0 |
|
 |
MES hydrate
CAS:1266615-59-1 |
|
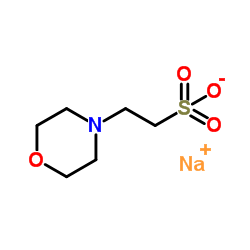 |
MES sodium salt
CAS:71119-23-8 |
|
 |
MES
CAS:4432-31-9 |