| Structure | Name/CAS No. | Articles |
|---|---|---|
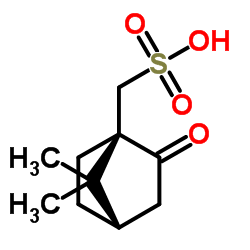 |
L(-)-Camphorsulfonic acid
CAS:35963-20-3 |
|
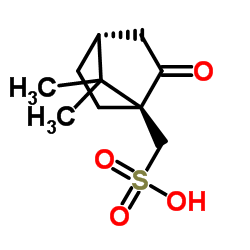 |
D-Camphorsulfonic acid
CAS:3144-16-9 |
|
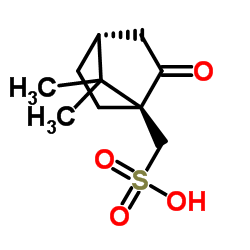 |
DL-10-Camphorsulfonic Acid
CAS:5872-08-2 |