Sequential ring-closing metathesis and nitrone cycloaddition on glucose-derived substrates: a divergent approach to analogues of spiroannulated carbanucleosides and conformationally locked nucleosides.
Sk Sahabuddin, Ashim Roy, Michael G B Drew, Biswajit Gopal Roy, Basudeb Achari, Sukhendu B Mandal
Index: J. Org. Chem. 71(16) , 5980-92, (2006)
Full Text: HTML
Abstract
The carbohydrate-derived substrate 3-C-allyl-1,2:5,6-di-O-isopropylidene-alpha-D-allofuranose was judiciously manipulated for preparing suitable synthons, which could be converted to a variety of isoxazolidino-spirocycles and -tricycles through the application of ring-closing metathesis (RCM) and intramolecular nitrone cycloaddition (INC) reactions. Cleavage of the isoxazolidine rings of some of these derivatives by transfer hydrogenolysis followed by coupling of the generated amino functionalities with 5-amino-4,6-dichloropyrimidine furnished the corresponding chloropyrimidine nucleosides, which were elaborated to spiroannulated carbanucleosides and conformationally locked bicyclo[2.2.1]heptane/oxa-bicyclo[3.2.1]octane nucleosides. However, use of higher temperature for the cyclization of one of the chloropyrimidines led to the dimethylaminopurine analogue as a sole product, formed via nucleophilic displacement of the chloro group by dimethylamine generated from DMF.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
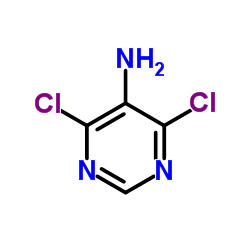 |
4,6-Dichloro-5-pyrimidinamine
CAS:5413-85-4 |
C4H3Cl2N3 |
|
6-(Alkylamino)-9-benzyl-9H-purines. A new class of anticonvu...
1988-03-01 [J. Med. Chem. 31(3) , 606-12, (1988)] |
|
An efficient and regiospecific strategy to N7-substituted pu...
2006-01-01 [J. Comb. Chem. 8(3) , 410-6, (2006)] |
|
Adenosine deaminase inhibitors. Synthesis and biological eva...
1992-10-30 [J. Med. Chem. 35(22) , 4180-4, (1992)] |
|
Synthesis of oxepane ring containing monocyclic, conformatio...
2007-09-14 [J. Org. Chem. 72(19) , 7427-30, (2007)] |
|
[Tetrahedron Lett. 48 , 1489, (2007)] |